Answer:
E = 7.99 *10^{-13} J
Step-by-step explanation:
the given reaction is
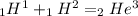
we know that energy is given as

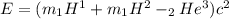
where
m_1 H^1 is mass of proton = 1.672622 *10^{-27}
m_1 H^2 is mass of deuterium = 3.344494 *10^{-27}
m_2 H^3 is mass of He = 5.008234 *10^{-27}
E = [1.672622 *10^{-27} + 3.344494 *10^{-27} - 5.008234 *10^{-27} ] *(3*10^8)^2
E = 7.99 *10^{-13} J