Answer:
a) [Cu(H2O)6]^2+
b) [CuCl4]^2-
Step-by-step explanation:
a) The energy(E) of a photon is related to its wavelength(λ) via the planck's equation:
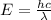
Therefore, energy and wavelength are inversely related.
The electromagnetic spectrum in the visible region extends from the high energy (low wavelength) blue end to the low energy (high wavelength) red region. For a given substance, absorption and emission are complementary to each other i.e. if the absorption occurs in the high energy region then photons will be emitted that corresponds to lower energy and vice versa. The wavelength region can be estimated based on the color wheel.
CuCl4^2- appears green, which implies that the absorption will occur in the red region
Cu(H2O)6^2+ appears blue which implies that the absorption will occur in the orange region
Red photons have a lower energy than the orange photons. Hence, Cu(H2O)6^2+ will absorb higher energy photons.
b) Copper (Cu) present in the given complexes is a transition metal with degenerate d -orbitals. Crystal field splitting removes the degeneracy of these 'metal' d-orbitals based on the strength of the ligands surrounding it.
As per the spectrochemical series, Cl is a strong field ligand compared to H2O. Hence, CuCl4^2- will have a larger splitting.