Answer:
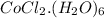 is formed and it has three unpaired electrons.
is formed and it has three unpaired electrons.
Step-by-step explanation:
Blue
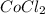 reacts with water to produce pink colored
reacts with water to produce pink colored
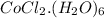 complex.
complex.
In this complex,
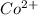 ion forms an octahedral complex with six
ion forms an octahedral complex with six
 ligands.
ligands.
As
 is a weak field ligand therefore
is a weak field ligand therefore
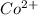 ion remains in low spin state.
ion remains in low spin state.
Hence electronic configuration of
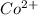 ion in this octahedral geometry is
ion in this octahedral geometry is
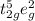 with total three unpaired electrons.
with total three unpaired electrons.