Step-by-step explanation:
According to Le Chatelier's principle, any disturbance occurring in an equilibrium reaction will shift the equilibrium in a direction that will oppose the change.
As the given reactions are as follows.
When concentration of
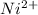 is increased in this reaction then reaction will shift in a direction that will be opposing the change. This means that the reaction will shift in backward direction.
is increased in this reaction then reaction will shift in a direction that will be opposing the change. This means that the reaction will shift in backward direction.
In this reaction, both water and ethylenediamine are neutral molecules. Hence, charge on nickel on each side will be equal to +2. Therefore, reaction will remain in equilibrium.
When we increase the concentration of
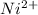 then equilibrium reaction will shift in the forward direction.
then equilibrium reaction will shift in the forward direction.
Here, on increasing the concentration of
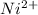 will shift the equilibrium in backward direction.
will shift the equilibrium in backward direction.
Thus, we can conclude that out of the given options
![[Ni^(2+)(aq) + 6NH_(3)(aq) \rightleftharpoons [Ni(NH_(3))_(6)]^(2+)(aq)](https://img.qammunity.org/2020/formulas/chemistry/college/d1c2qia21vybgkqjotkco8lbu5pbvkju58.png) is the chemical reaction in which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of
is the chemical reaction in which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of
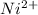 is increased.
is increased.