Answer:
The salt is barium chloride.
Step-by-step explanation:
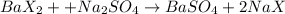
Moles of barium sulfate =
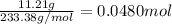
According to reaction, 1 mol of barium sulfate is produced from 1 mol of
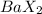 .
.
Then 0.0480 moles will be produced from:
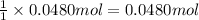 of
of
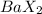 .
.
Mass of
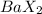 used = 10.00 g
used = 10.00 g
Moles of
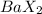 =\frac{10.00 g}{\text{Molar mass}}[/tex]
=\frac{10.00 g}{\text{Molar mass}}[/tex]
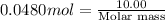
Molar mass of
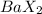 = 208.33 g/mol
= 208.33 g/mol
The nearest answer to our answer is
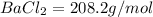 .
.
The correct answer barium chloride with molar mass of 208.2 g/mol.