Answer: c) 100.90°C
Step-by-step explanation:
Elevation in boiling point is given by:
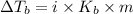
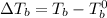 = elevation in boiling point
= elevation in boiling point
i= vant hoff factor = 2 (for NaI[/tex]
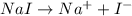
 = boiling point constant =
= boiling point constant =
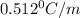
m= molality
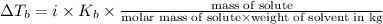
Weight of solvent i kg =48.6 g= 0.0486kg
![\Delta T_b=2* 0.512* \frac{6.37g}{150g/mol* 0.0486]()
![\Delta T_b=2* 0.512* \frac{6.37g}{150g/mol* 0.0486]()
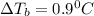
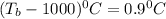
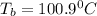
Thus the boiling point of the solution will be
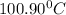 .
.