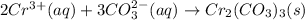Step-by-step explanation:
An ionic equation will be the one in which all the participating species will be present as ions.
The given reaction will be as follows.
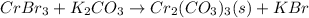
Balancing this equation by multiplying
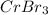 by 2 and
by 2 and
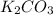 by 3 on reactant side. Whereas multiply KBr by 6 on product side.
by 3 on reactant side. Whereas multiply KBr by 6 on product side.
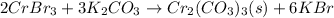
Hence, the net ionic equation will be as follows.
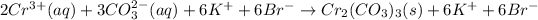
As both
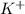 and
and
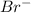 are spectator ions. Hence, the net ionic equation will be as follows.
are spectator ions. Hence, the net ionic equation will be as follows.