Step-by-step explanation:
Suppose the solubility of
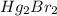 is x. Hence, upon dissociation equilibrium reaction for
is x. Hence, upon dissociation equilibrium reaction for
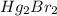 will be as follows.
will be as follows.
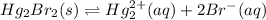
At equilibrium: x 2x
Therefore, equation for
 will be as follows.
will be as follows.
 =
=
![[Hg^(2+)_(2)][Br^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/zl6t6i9fara0u86wd4z3jzrlthf90vt80i.png)
=
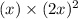
=
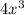
x =
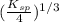
x =
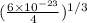
=
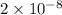
Thus, we can conclude that the water solubility of
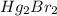 is
is
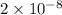 .
.