Answer: Option (d) is the correct answer.
Step-by-step explanation:
When a hydrogen atom comes in contact with an electronegative atom then it results in the formation of a chemical bond.
More is the electronegativity of combining atom, more stronger will be the bond with hydrogen atom. As a result, the compound formed will not easily give up hydrogen atom upon dissociation.
Whereas less is the electronegativity of atom combining with hydrogen atom, easily it will donate the hydrogen atom upon dissociation.
Since, out of the given option sulfur (S) atom has low electronegativity as compared to oxygen and nitrogen atom.
Hence,
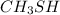 will easily donate hydrogen atom.
will easily donate hydrogen atom.
Thus, we can conclude that
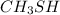 molecule would be the best hydrogen bond donor.
molecule would be the best hydrogen bond donor.