Answer :
The Nernst equation :
![E_(cell)=E^o_(cell)-(2.303RT)/(nF)\log ([Anode])/([Cathode])](https://img.qammunity.org/2020/formulas/chemistry/college/krr5tcayntq69n9e0at718kmd3ykznpoa7.png)
where,
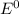 = standard cell potential
= standard cell potential
n = number of electrons in oxidation-reduction reaction
F = Faraday constant = 96500 C
R= gas constant = 8.314 J/Kmol
T = temperature
[Anode] = anodic ion concentration
[Cathode] = cathodic ion concentration