Answer:
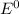 for
for
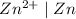 system would be -1.53 V
system would be -1.53 V
Step-by-step explanation:
Determination of standard reduction potential of any system is done by placing the system in cathode and a reference half cell in anode and then evaluate the cell potential. The cell potential is the standard reduction potential of the system.
So
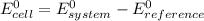
As
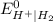 is equal to 0 therefore cell potential is equal to reduction potential of any system by taking hydrogen electrode as a reference.
is equal to 0 therefore cell potential is equal to reduction potential of any system by taking hydrogen electrode as a reference.
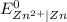 equal to -0.76 V with respect to hydrogen
equal to -0.76 V with respect to hydrogen
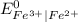 equal to 0.77 V with respect to hydrogen
equal to 0.77 V with respect to hydrogen
Therefore standard reduction potential of
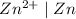 system when
system when
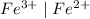 system is taken as reference is-
system is taken as reference is-
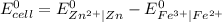
= -0.76 V - 0.77 V
= -1.53 V