Answer:
The volume of NaOH required will be 33.33 mL.
0.48 is the pH of a 0.33 M solution of HCI.
Step-by-step explanation:
1)
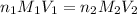
where,
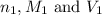 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is

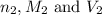 are the n-factor, molarity and volume of base which is HCl.
are the n-factor, molarity and volume of base which is HCl.
We are given:
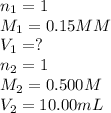
Putting values in above equation, we get:
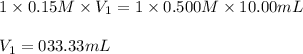
The volume of NaOH required will be 33.33 mL.
2) The pH of the solution is defined as negative logarithm of hydrogen ion's concentration in a solution.
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
![pH=-\log[0.33 M]=0.48](https://img.qammunity.org/2020/formulas/chemistry/college/p0i7fvjmyrgnt3b2rrvhviyjzfc65n1mt8.png)
0.48 is the pH of a 0.33 M solution of HCI.