Answer: Option (b) is the correct answer.
Step-by-step explanation:
Relation between
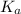 and
and
 is as follows.
is as follows.
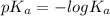
where,
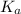 is the acid dissociation constant.
is the acid dissociation constant.
As value of
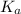 is given as
is given as
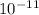 . Therefore, calculating teh value of
. Therefore, calculating teh value of
 as follows.
as follows.
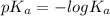
= -log (
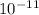 )
)
= - (-11 × log (10))
= 11
Thus, we can conclude that the
 of an acid whose
of an acid whose
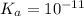 is 11.
is 11.