Answer: The mass percent of
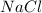 is 6.3 % and mass percent of water is 93.7%.
is 6.3 % and mass percent of water is 93.7%.
Step-by-step explanation:
To calculate the mass percent of element in a given compound, we use the formula:
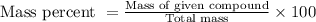
Given :
Mass of
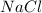 = 13.5 g
= 13.5 g
Mass of water = 250.0 g
Putting values in above equation, we get:
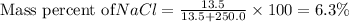
Hence, the mass percent of
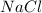 is 6.3 %.
is 6.3 %.
Putting values in above equation, we get:
![\text{Mass percent of water=(200)/(13.5+250.0)* 100=93.7\%]()
Hence, the mass percent of water is 93.7 %.