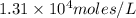Answer:
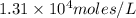
Step-by-step explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as

The equation for the ionization of the silver chromate is given as:
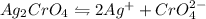
We are given:
Solubility of
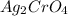 = S mol/L
= S mol/L
By stoichiometry of the reaction:
1 mole of
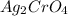 gives 2 moles of
gives 2 moles of
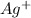 and 1 mole of
and 1 mole of
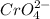 .
.
When the solubility of
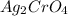 is S moles/liter, then the solubility of
is S moles/liter, then the solubility of
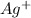 will be 2S moles\liter and solubility of
will be 2S moles\liter and solubility of
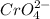 will be S moles/liter.
will be S moles/liter.
Expression for the equilibrium constant of
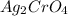 will be:
will be:
![K_(sp)=[Ag^+]^2[CrO_4^(2-)]](https://img.qammunity.org/2020/formulas/chemistry/college/y771i2zpyqx8prs03p9mtrlh8htk7roixc.png)
![9.00* 10^(12)=[2s]^2[s]=4s^3](https://img.qammunity.org/2020/formulas/chemistry/college/nf0ti409etuk5mzrp46b9nt48hjcaefzjy.png)
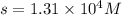
Hence, the solubility of
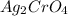 is
is