Answer: Option (B) is the correct answer.
Step-by-step explanation:
An aqueous solution that consists of a mixture of weak base and its conjugate acid or a weak acid and its conjugate base is known as a buffer.
When we add a strong acid or base into this mixture then the change in pH of this mixture is very small.
In a buffer solution, pH depends on the ratio of concentration of base divided by concentration of acid.
It is known that for a given temperature, the value of
 remains constant.
remains constant.
Therefore, relation between pH and
 will be as follows.
will be as follows.
pH =
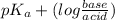
Thus, we can conclude that the purpose of an acid-base buffer is to maintain a relatively constant pH in a solution.