Step-by-step explanation:
The given reaction will be as follows.
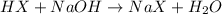
Initial : 2.2 1.15 0 0
At equili:(2.2 - 1.15) 0 1.15 1.15
As moles of [HX] = (2.2 - 1.15) = 1.05
Moles of [NaX] = 1.15
So, relation between pH and
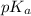 will be as follows.
will be as follows.
pH =
![pK_(a) + (log[NaX])/([HX])](https://img.qammunity.org/2020/formulas/chemistry/college/6ziz98x5ulop3fir78wedl8lzrvg17ht96.png)
![pK_(a) = pH - log (log[NaX])/([HX])](https://img.qammunity.org/2020/formulas/chemistry/college/e05w1riqo4lxneyvke05zcabn12hxag35k.png)
=
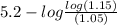
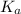 =
=
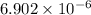
Thus, we can conclude that
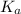 for the acid HX is
for the acid HX is
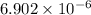 .
.