Answer:
For a: The reactions are written below.
For b: The standard cell potential of the given cell is +0.44 V.
For c: The cell potential for the given values is 0.408 V
For d: The cell potential for the given values is 0.4724 V.
Step-by-step explanation:
The given cell is:
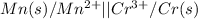
Half reactions for the given cell follows:
Oxidation half reaction:
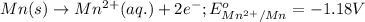 ( × 3)
( × 3)
Reduction half reaction:
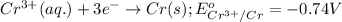 ( × 2)
( × 2)
Net reaction:
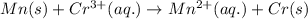
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
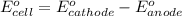
Putting values in above equation, we get:

Hence, the standard cell potential of the given cell is 0.44 V
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Mn^(2+)])/([Cr^(3+)])](https://img.qammunity.org/2020/formulas/chemistry/college/9zzgzniggy2uv7idprqxwkferdpan6fx5z.png)
where,
 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V
 = standard electrode potential of the cell = +0.44 V
= standard electrode potential of the cell = +0.44 V
n = number of electrons exchanged = 6
![[Cr^(3+)]=1.0* 10^(-4)M](https://img.qammunity.org/2020/formulas/chemistry/college/pptsdf1wr1tm87jdrwa4p7i5qxfg3q9vjq.png)
![[Mn^(2+)]=0.20M](https://img.qammunity.org/2020/formulas/chemistry/college/priedua1xyrgc2bkn2oonqbhs9kp2ytcq0.png)
Putting values in above equation, we get:
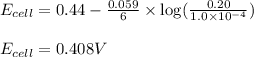
Hence, the EMF of the cell for the given concentration is 0.408 V.
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Mn^(2+)])/([Cr^(3+)])](https://img.qammunity.org/2020/formulas/chemistry/college/9zzgzniggy2uv7idprqxwkferdpan6fx5z.png)
where,
 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V
 = standard electrode potential of the cell = +0.44 V
= standard electrode potential of the cell = +0.44 V
n = number of electrons exchanged = 6
![[Cr^(3+)]=0.20M](https://img.qammunity.org/2020/formulas/chemistry/college/fz07zu7aeldtzldcgnmiknunggnyzlfbir.png)
![[Mn^(2+)]=1.0* 10^(-4)M](https://img.qammunity.org/2020/formulas/chemistry/college/1d6rry994gfs7e07fdfgcf00gtj83o5ci5.png)
Putting values in above equation, we get:
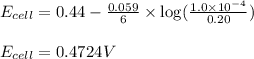
Hence, the EMF of the cell for the given concentration is 0.4724 V.