Step-by-step explanation:
It is given that the total volume is (10 mL + 60 mL) = 70 mL.
Also, it is known that
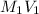 =
=
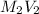
Where,
 = total volume
= total volume
 = initial volume
= initial volume
Therefore, new concentration of
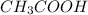 =
=
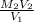
=
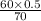
= 0.43 M
New concentration of NaOH =
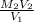
=
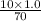
= 0.14 M
So, the given reaction will be as follows.
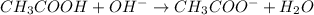
Initial: 0.43 0.14 0
Change: -0.14 -0.14 0.14
Equilibrium: 0.29 0 0.14
As it is known that value of
 = 4.74
= 4.74
Therefore, according to Henderson-Hasselbalch equation calculate the pH as follows.
pH =
![pK_(a) + log ([CH_(3)COO^(-)])/([CH_(3)COOH])](https://img.qammunity.org/2020/formulas/chemistry/college/j2enp89t2cfkj13gg4j736k2itay9e6scb.png)
=
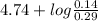
= 4.74 + (-0.316)
= 4.42
Therefore, we can conclude that the pH of given reaction is 4.42.