Answer:
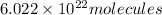 of dimethylhydrazine are present in 0.100 mol.
of dimethylhydrazine are present in 0.100 mol.
Step-by-step explanation:
Moles of dimethylhydrazine ,n= 0.100 mol
Molecules = Moles × Avogadro number =
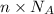
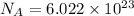
Number of molecules of of dimethylhydrazine:
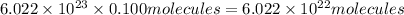
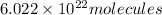 of dimethylhydrazine are present in 0.100 mol.
of dimethylhydrazine are present in 0.100 mol.