Answer: The pH is 1.69897 and the solution is acid.
Step-by-step explanation:
The complete neutralization reaction will be:
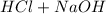 →
→
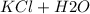
In consequence, if we add 0.03 mol of HCl and 0.01 mol of NaOH, we are going to have an excess of 0.02 mol of HCl that is not going to react. Further, the resulting solution will have an acidic character.
On the other hand, for obtaining the pH, we use the formula:
![pH=-log[H+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/fo8ntyunv90lqa0v7jzlmxegemfb3owf2z.png)
Where [H+] is expressed in molar concentration.
As HCl is a strong acid, when it is dissolved the H+ ions are completely dissociated and the concentration of HCl in excess is the same of H+ ions.
Before using the formula, we have to calculate the molar concentration of H+ ions, dividing 0.02 mol (HCl in excess) between 1 L; so the concentration of H+ ions is 0.02 M.
Finally, the pH will be:
![pH=-log[0.02]=1.69897](https://img.qammunity.org/2020/formulas/chemistry/high-school/ulg8bcsreldr52xmsjjsinak3dccmtrgj8.png)