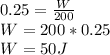Answer:
Step-by-step explanation:
The work done
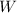 and the energy taken
and the energy taken
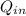 by a heat engine are related to the efficiency
by a heat engine are related to the efficiency
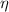 by the expression
by the expression
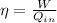
The efficiency is
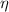 =25%, the numerical form of this percentage is 0.25 and the energy taken is
=25%, the numerical form of this percentage is 0.25 and the energy taken is
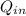 =200J. Replacing in the formula:
=200J. Replacing in the formula: