Answer:


Step-by-step explanation:
Given that
Power produces=200 KW
Power input=225 KW
We know that from first law of thermodynamics

Where
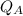 heat in put
heat in put
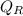 heat rejection
heat rejection
W is the power output
So now putting the values


We know that thermal efficiency given as



Now to find the possibility of such type of engine .we will find the Carnot efficiency at given temperature and then will compare will obtain above efficiency of engine.
We know that Carnot efficiency is the maximum possible efficiency of any type work producing devices.No any device will have efficiency more than Carnot efficiency .If any device will have more than Carnot efficiency then it device will not possible.
So Carnot efficiency



So this device is not possible because it have efficiency more than Carnot efficiency.This is not reasonable.