Answer:
K I will attempt
Step-by-step explanation:
a)
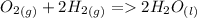
b)
1 : 2 : 2 (I don't know if this is what the question wants but it is what I would answer)
c)
Hydrogen because it requires 2 moles of H2 to react with 1 mole of O2
d)
24 moles of water. Look at stoichiometric coefficient. 2:2 means 24 moles you get 24 moles
e)
Oxygen. 2 < 5/2. Remember, 1 mole of O2 requires 2 moles of H2. But 5/2 is still greater than 2
f)
First, let's find out how many moles of water we can get. Since O2 is the limiting reactant, and O2:H2O ratio is 1:2, we will get 4 moles of H2O. Then, we can multiply 4 by Avogadro's number which is
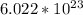 to get the number of molecules. We get: 2.41 * 10^24 molecules of water.
to get the number of molecules. We get: 2.41 * 10^24 molecules of water.