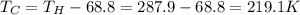(a) 287.9 K
The efficiency of the engine is given by
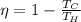
where
Tc is the temperature of the cold reservoir
Th is the temperature of the hot reservoir
We can rewrite the two temperatures as
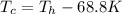
(because differences in Kelvin are equal to differences in Celsius)
So we can rewrite the 1st equation as
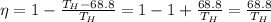
We also know the efficiency:
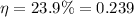
So we can re-arrange the previous equation to find the temperature of the hot reservoir:
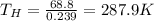
(b) 219.1 K
The temperature of the cold reservoir can be found as: