Answer:
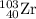 , zirconium-103.
, zirconium-103.
Step-by-step explanation:
In a nuclear reaction, both the mass number and atomic number will conserve.
Let
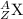 represent the unknown particle.
represent the unknown particle.
The mass number of a particle is the number on the upper-left corner. The atomic number of a particle is the number on its lower-left corner under the mass number. For example, for the particle
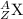 ,
,
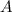 is the mass number while
is the mass number while
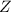 while
while
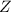 is the atomic number.
is the atomic number.
Sum of mass numbers on the left-hand side of the equation:
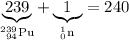 .
.
Note that there are three neutrons on the right-hand side of the equation. Sum of mass numbers on the right-hand side:
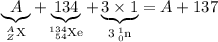 .
.
Mass number conserves. As a result,
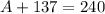 .
.
Solve this equation for
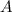 :
:
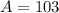 .
.
Among the five choices, the only particle with a mass number of 103 is
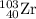 . Make sure that atomic number also conserves.
. Make sure that atomic number also conserves.