Answer:
Here's what I get
Step-by-step explanation:
Solubility rules
- Salts containing halides are generally soluble. Important exceptions to this rule are halides of silver, mercury, and lead(II).
- All acetates, chlorates, and perchlorates are soluble
So, PbCl₂ is insoluble, and Pb(ClO₃)₂ is soluble.
1. "Molecular" equation
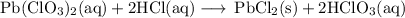
2. Ionic equation
Convert the soluble salts to their hydrated ions.
HCl and HClO₃ are strong acids. Convert them to their ions.

3. Net ionic equation
Cancel all ions that appear on both sides of the reaction arrow (in boldface).
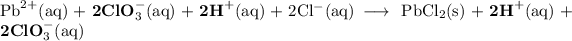
The net ionic equation is
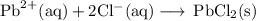
4. Theoretical yield
We have the volumes and concentrations of two reactants, so this is a limiting reactant problem.
We know that we will need a balanced equation with masses, moles, and molar masses of the compounds involved.
(i). Gather all the information in one place with molar masses above the formulas and masses below them.
M_r: 278.11
Pb(ClO₃)₂ + 2HCl ⟶ PbCl₂ + 2HClO₃
Volume/mL: 125 95
c/mol·L⁻¹: 0.85 0.85
(ii) Calculate the moles of each reactant
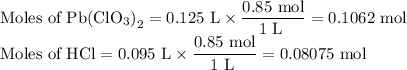
(iii) Identify the limiting reactant
Calculate the moles of PbCl₂ we can obtain from each reactant.
From Pb(ClO₃)₂:
The molar ratio of PbCl₂:Pb(ClO₃)₂ is 2:2
Moles of PbCl₂ = 0.1062 × 2/2 =0.1062 mol PbCl₂
From HCl :
The molar ratio of PbCl₂:HCl is 1 mol PbCl₂:2 mol HCl.
Moles of PbCl₂ = 0.08075 × 1/2 = 0.04038 mol PbCl₂
The limiting reactant is HCl because it gives the smaller amount of PbCl₂.
(iv) Calculate the theoretical yield of PbCl₂.
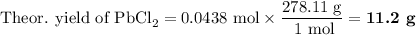
5. Calculate the actual yield of PbCl₂
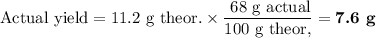
6. Calculate [ClO₃⁻]
Original concentration of Pb(ClO₃)₂ = 0.85 mol·L⁻¹
Original concentration of ClO₃ = 2 × 0.85 = 1.70 mol·L⁻¹
The solution was diluted by the addition of HCl.
Total volume = 125 + 95 =220 mL
c₁V₁ = c₂V₂
1.70 mol·L⁻¹ × 125 mL = c₂ × 220 mL
212.5 mol·L⁻¹ = 200 c₂
c₂ = (212.5 mL)/200 = 1.06 mol·L⁻¹
7. Calculate [Pb²⁺].
Moles of Pb²⁺ originally present = 0.1062 mol
Moles of Pb²⁺removed = 0.04038 mol
Moles of Pb²⁺ remaining = 0.0659 mol
c = 0.0659 mol/0.220 L = 0.299 mol·L⁻¹