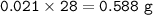Total moles of gas = 0.1225
Volume of gas produced : 6.7375 L
mass of Nitrogen : 0.588 g
Further explanation
Given
2 ml of Nitroglycerin(ρ=1.592 g/ml)
Required
Total moles of gas
Solution
Nitroglycerin detonated ⇒ decomposition reaction
4C₃H₅N₃O₉(s)⇒ 6N₂(g)+12CO(g)+10H₂O(g)+7O₂(g)
mass of Nitroglycerin :
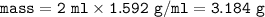
moles of Nitroglycerin :
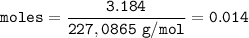
Total moles of gas:
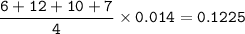
Volume of gas produced :
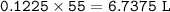
moles of Nitrogen :
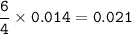
mass of Nitrogen :