Step-by-step explanation:
The given data is as follows.
Thickness = 0.28 mm =
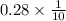 cm = 0.028 cm
cm = 0.028 cm
Area = 0.40
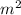 =
=
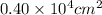 = 4000
= 4000
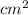
As, it is known that volume = area × thickness
So, Volume =

= 112

As density is mass divided by volume. So, mass of chromium will be calculated as follows.
Density =

7.20
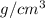 =
=
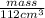
mass = 806.4 g
As, mass of 1 mole of chromium is 52 g. So, number of moles in 806.4 g of chromium will be as follows.
No. of moles =
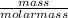
=
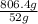
= 15.50 mol
In chromate ion, (
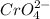 ) charge on Cr is +6. It means that 6 electrons are needed to reduce
) charge on Cr is +6. It means that 6 electrons are needed to reduce
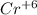 into Cr.
into Cr.
As, 1 mole of
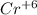 ions require 6 moles of electrons. Therefore, moles of electrons for 15.50 mol will be calculated as follows.
ions require 6 moles of electrons. Therefore, moles of electrons for 15.50 mol will be calculated as follows.
6 × 15.50 mol = 93.04 mol
To calculate number of electrons we multiply number of moles by Avogadro's number as follows.
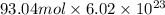
=
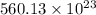
=
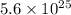 electrons
electrons
There is magnitude of
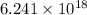 times the charge on an electron is equal to 1 coulomb.
times the charge on an electron is equal to 1 coulomb.
Hence, number of coulombs will be as follows.
No. of coulombs =
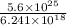
=
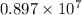 C
C
or, =
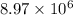 C
C
Thus, we can conclude that
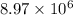 C are required to plate a layer of chromium metal with given data.
C are required to plate a layer of chromium metal with given data.