Answer: magnesium hydroxide
Step-by-step explanation:
According to Arrhenius concept, a base is defined as a substance which donates hydroxide ions
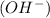 when dissolved in water and an acid is defined as a substance which donates hydronium ions
when dissolved in water and an acid is defined as a substance which donates hydronium ions
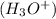 in water.
in water.
Magnesium sulfate has chemical formula
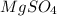
Magnesium chloride has chemical formula
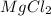
Magnesium hydroxide has chemical formula
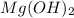
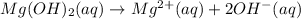
Magnesium acetate has chemical formula
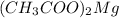
Thus magnesium hydroxide is a base when dissolved in water, as defined by Arrhenius.