Step-by-step explanation:
Given that,
Wavelength = 6.0 nm
de Broglie wavelength = 6.0 nm
(a). We need to calculate the energy of photon
Using formula of energy
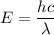
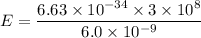
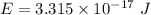
(b). We need to calculate the kinetic energy of an electron
Using formula of kinetic energy
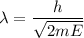
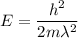
Put the value into the formula
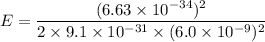
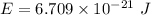
(c). We need to calculate the energy of photon
Using formula of energy
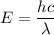
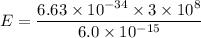
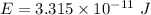
(d). We need to calculate the kinetic energy of an electron
Using formula of kinetic energy
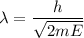
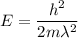
Put the value into the formula
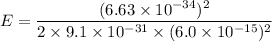
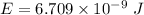
Hence, This is the required solution.