Answer:
Methane gas is formed in this reaction. After adding aqueous acid, ethyl acetoacetate is reformed through protonation of it.
Explanation:
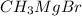 acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.
acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.
Hence
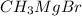 abstracts a proton from methylene group and being converted to methane gas.
abstracts a proton from methylene group and being converted to methane gas.
After addition of aqueous acid, conjugate base of ethyl acetoacetate gets protonated and ethyl acetoacetate is reproduced.
See the attachment below.