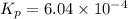Answer:
The value of equilibrium constant is :
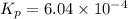
Step-by-step explanation:
Partial pressure of nitrogen at equilibrium,
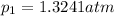
Partial pressure of oxygen at equilibrium,
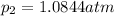
Partial pressure of dinitrogen monoxide at equilibrium,
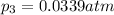
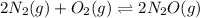
The expression of
 will be given as:
will be given as:
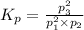
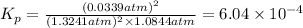
The value of equilibrium constant is :