Answer: Option (D) is the correct answer.
Step-by-step explanation:
It is known that molecular formula of water is
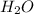 . And, due to difference in electronegativity of both hydrogen and oxygen atom there will be a partial positive charge on hydrogen atom and a partial negative charge on oxygen atom.
. And, due to difference in electronegativity of both hydrogen and oxygen atom there will be a partial positive charge on hydrogen atom and a partial negative charge on oxygen atom.
The compound KCl is ionic in nature as it is formed by transfer of electron from potassium to chlorine atom forming an ionic bond between the two.
So, when KCl is added to water then being polar or ionic in nature KCl will dissociate into ions as
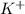 and
and
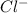 . As oxygen atom of water has partial positive charge so it will attract
. As oxygen atom of water has partial positive charge so it will attract
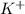 ions towards itself.
ions towards itself.
And,
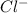 ions will be attracted towards the partial positive charge of hydrogen atom of water.
ions will be attracted towards the partial positive charge of hydrogen atom of water.
Thus, we can conclude that when KCl dissolves in water the
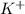 ions are attracted to the partially negative oxygen atoms of the water molecules.
ions are attracted to the partially negative oxygen atoms of the water molecules.