Answer:
food value of the peanut is 38 000 cal
Step-by-step explanation:
Heat observed by water
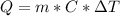
C is a constant.
For water, C = 1 c gm/ °C
So here Q = (1000) (1 ) (47-28)
Q = 19 000 cal
FOR 50% efficiency,
Total heat released by peanut is:
19,000 cal / 0.50 = 38 000 cal
So the food value of the peanut is 38 000 cal.