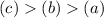Answer:
Most viscous to least viscous:
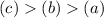
Step-by-step explanation:
For hydrocarbons, viscosity increases with increasing molar mass. Because increasing molar mass signifies increase in number of electrons in molecules.
We know that in non-polar hydrocarbons, only van der waal intermolecular force exists. Van der waal force is proportional to number of electrons in a molecule.
Therefore with increasing molar mass, van der waal force increases. hence molecules gets more tightly bind with each other resulting increase in viscosity.
Here molar mass order :
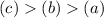
Therefore viscosity order :