Answer:
the molality of the solution is 10 mol/kg
Step-by-step explanation:
To find the molality of a solution we apply the following formula
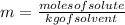
data:
100 g of solvente
We have the amount of solvent expressed in grams but to be able to use the data in the formula we must pass it to kilograms
we apply a simple rule of three
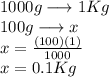
27.1g of solute, molar mass 27.1 g/mol
We need the moles of solute and they give us grams of solute and molar mass
The molar mass is 27.1 g / mol This means that there is 27.1 g for each mol of solute.
The amount of solute we have is 27.1g and according to the molar mass this amount of solute corresponds exactly to 1 mole
Therefore having 27.1 g of solute is equivalent to saying that we have 1 mol of solute
Now that we have the data in the correct units we apply the formula
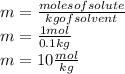
the molality of the solution is 10 mol/kg