Answer:
1-propanol --> propanone --> pentane --> methane
Step-by-step explanation:
Hello,
Vapor pressure is referred to the pressure exerted by the vapor that in equilibrium with a substance liquid. In organic compounds, such vapor pressure is higher as the number of carbons is lower since the intermolecular forces become weaker, nevertheless, the presence of functional groups modify this behavior by means of the intermolecular forces magnitudes (weaker intermolecular forces imply higher vapor pressures as the particles tend to separate to each other), thus, by searching on the NIST webpage for their vapor pressures at 25°C, one finds:
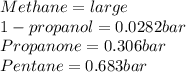
In such a way, the lowest vapor pressure belongs to 1-propanol, next propanone, then pentane and finally methane.
Best regards.