Answer: The number of moles of HCl that will react with given amount of Fe is 9.2 moles.
Step-by-step explanation:
We are given:
Moles of iron = 4.6 moles
For the given chemical reaction:
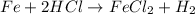
By Stoichiometry of the reaction:
1 mole of iron reacts with 2 moles of hydrochloric acid.
So, 4.6 moles of iron will react with =
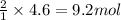 of hydrochloric acid.
of hydrochloric acid.
Hence, the number of moles of HCl that will react with given amount of Fe is 9.2 moles.