Answer : The moles of
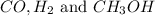 are, 0.1187, 0.2374 and 0.0313 moles respectively.
are, 0.1187, 0.2374 and 0.0313 moles respectively.
Explanation : Given,
Initial moles of
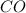 = 0.15 mole
= 0.15 mole
Initial moles of
 = 0.3 mole
= 0.3 mole
Moles of
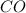 at equilibrium = 0.1187 mole
at equilibrium = 0.1187 mole
The given equilibrium reaction is,

Initial moles 0.15 0.3 0
At equilibrium (0.15-x) (0.3-2x) x
As we are given that,
Moles of
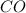 at equilibrium = 0.1187 mole = 0.15 - x
at equilibrium = 0.1187 mole = 0.15 - x
0.15 - x = 0.1187
x = 0.0313
Moles of
 at equilibrium = 0.3 - 2x = 0.3 - 2(0.0313) = 0.2374 mole
at equilibrium = 0.3 - 2x = 0.3 - 2(0.0313) = 0.2374 mole
Moles of
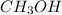 at equilibrium = x = 0.0313 mole
at equilibrium = x = 0.0313 mole
Therefore, the moles of
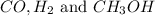 are, 0.1187, 0.2374 and 0.0313 moles respectively.
are, 0.1187, 0.2374 and 0.0313 moles respectively.