Answer: The
 for the reaction is 54.6 kJ/mol
for the reaction is 54.6 kJ/mol
Step-by-step explanation:
For the given balanced chemical equation:
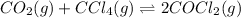
We are given:
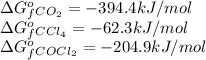
- To calculate
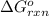 for the reaction, we use the equation:
for the reaction, we use the equation:
![\Delta G^o_(rxn)=\sum [n* \Delta G_f(product)]-\sum [n* \Delta G_f(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/college/7zgd3o9ob4ygweyguu3qejxhr42jvq26wr.png)
For the given equation:
![\Delta G^o_(rxn)=[(2* \Delta G^o_f_((COCl_2)))]-[(1* \Delta G^o_f_((CO_2)))+(1* \Delta G^o_f_((CCl_4)))]](https://img.qammunity.org/2020/formulas/chemistry/college/ruwesg13zb83aea4z5qrfkom1k1rqh8d2i.png)
Putting values in above equation, we get:
![\Delta G^o_(rxn)=[(2* (-204.9))-((1* (-394.4))+(1* (-62.3)))]\\\Delta G^o_(rxn)=46.9kJ=46900J](https://img.qammunity.org/2020/formulas/chemistry/college/3pwknddl2xj7yyif7c20znwcj9cv1tu80r.png)
Conversion factor used = 1 kJ = 1000 J
- The expression of
 for the given reaction:
for the given reaction:
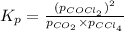
We are given:
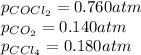
Putting values in above equation, we get:
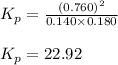
- To calculate the Gibbs free energy of the reaction, we use the equation:
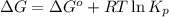
where,
 = Gibbs' free energy of the reaction = ?
= Gibbs' free energy of the reaction = ?
 = Standard gibbs' free energy change of the reaction = 46900 J
= Standard gibbs' free energy change of the reaction = 46900 J
R = Gas constant =
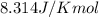
T = Temperature =
![25^oC=[25+273]K=298K](https://img.qammunity.org/2020/formulas/physics/high-school/h3swi627jfkpg7vx7in8p5pe35bz1gwehq.png)
 = equilibrium constant in terms of partial pressure = 22.92
= equilibrium constant in terms of partial pressure = 22.92
Putting values in above equation, we get:
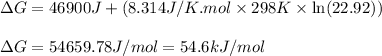
Hence, the
 for the reaction is 54.6 kJ/mol
for the reaction is 54.6 kJ/mol