Answer:
0.1000M
Step-by-step explanation:
Given parameters:
Volume of acid = 20mL, to liter gives 2 x 10⁻²L
Volume of NaOH = 43mL to liter gives 4.3 x 10⁻²L
Molarity of NaOH = 0.1M
Unknown:
Molarity of acid = ?
Solution;
The acid is easily identified as a carboxylic acid which is called ethanoic acid. With the identification of the acid, we can write the reaction equation as shown below:
CH₃COOH + NaOH → CH₃COONa + H₂
Now that we have established the reaction equation, we proceed to solve for molarity of the acid given.
To solve for the molarity of the acid, we solve from the known parameters to the unknown ones. We know the details of the base NaOH used.
We first find the number of moles of NaOH as shown below:
Number of moles of NaOH = molarity x volume of NaOH
Number of moles of NaOH = 0.1 x 4.3 x 10⁻² = 0.0043mole
From the reaction equation, we know that:
1 mole of NaOH reacted with 1 mole of ethanoic acid
0.0043mole of NaOH will produce 0.0043mole of the acid
Now to find the molarity of the acid, we use the expression below:
Molarity of acid =
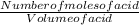
Molarity of acid =
 = 0.1M
= 0.1M