Answer:
- Because it has or not the same number of atoms at each side.
- Because the mass of reactants and product is or not the same after the reaction.
Step-by-step explanation:
Hello!
In this case, since the law of conservation of mass states that matter cannot be created nor destroyed; when we see a chemical reaction, we need to make sure that the amount of atoms is the same at both reactants and products; for instance the formation of water requires four hydrogen atoms and two oxygen atoms at both reactants and products sides as shown below:
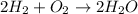
Another way is by proving that the total mass of products equals the total mass of reactants once the reaction has gone to completion.
Best regards!