Answer: The correct answer is Option b.
Step-by-step explanation:
An acid is defined as the substance which looses hydrogen ions when dissolved in water.
A strong acid is defined as the substance which gets completely dissociated into its ions when dissolved in water. Their percentage ionization is equal to 100 %. For Example:
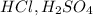 etc.. They can be monoprotic, diprotic or tripotic.
etc.. They can be monoprotic, diprotic or tripotic.
A weak acid is defined as the substance which does not get completely dissociated into its ions when dissolved in water. Their percentage ionization is less than 100 %. For Example:
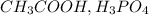 etc.. They can be monoprotic, diprotic or tripotic.
etc.. They can be monoprotic, diprotic or tripotic.
Hence, the correct answer is Option b.