Answer:12,352 cal
Step-by-step explanation:
Given
to change 16 gm ice at
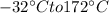
First we need to take ice to
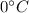 then change its state to water and then raises water temperature from 0 to
then change its state to water and then raises water temperature from 0 to
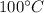 .
.
After it change water phase to steam and then raise its temperature to
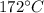
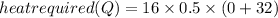 +
+
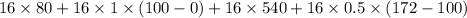
Q=12,352 cal