Answer:
The soultion of pOH=9
Step-by-step explanation:
We can determine the acidity or the basicity of the solution using the pOH value.
The solution is acidic if the pOH value ranges from 7.1 to 14
The solution is Basic if the pOH value ranges from 0 to 6.9
The solution is neutral if the pOH value is 7.
pOH is defined as the negative logarithm of the Hydroxide ion concentration, that’s why the formula to find pOH is given by
![pOH=-log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/zqwq92ac1oz8gijorova3gwi6v130yj3ld.png)
![pOH=-log[1.00*10^(-9)M]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/p66fixz299cbmd9mhexlxmnzyf0hwf2w5i.png)
![=-[-9]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/9ils648wu4dc128881oxqte916j7g3tcd9.png)
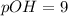
(Answer)
Please note :
A Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples
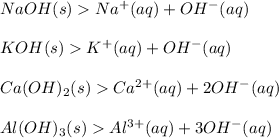
Please note:
(aq) stands for aqueous which means in the presence of water that is,water acts as a solvent
So, on adding a base to the water increase in
![[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xxde7ud270ullhgkdtd9pogar35adf8qg0.png) will take place and this will decrease the Hydrogen ion concentration
will take place and this will decrease the Hydrogen ion concentration
Pure water contains
![[H^+ ]=[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/sbe7a2aeootq2epsmun4y7bkdsa5y67ad1.png)
if the solution is acidic
![[H^+ ]>[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/td2rwjaxkogn9dkqmg7p5la1hojsi7vycc.png)
if the solution is Basic
![[H^+ ]<[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/wflkjtk5b2lxhn25uo9sskbq195l5hle10.png)