Answer: The correct answer is Option D.
Step-by-step explanation:
Ionic compounds are defined as the compounds which are formed by the complete transfer of electrons from one atom to another atom. In these compounds, oppositely charged ions are attracted towards each other to form a compound.
Hydrogen is the 1st element of the periodic table having 1 electron. The electronic configuration of this element is
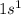
This will loose 1 electron to form
 ion.
ion.
Chlorine is the 17th element of the periodic table having 17 electrons. The electronic configuration of this element is
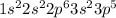
This will gain 1 electron to form
 ion.
ion.
To form HCl compound, 1 hydrogen ion is needed to neutralize the charge on chlorine ion.
Hence, the correct answer is Option D.