Answer:
1.25 is the pH of the given solution
Step-by-step explanation:
We can determine the acidity or the basicity of the solution using the pH value.
The solution is acidic if the pH value ranges from 0 to 6.9
The solution is Basic if the pH value ranges from 7.1 to 14
The solution is neutral if the pH value is 7.
pH is defined as the negative logarithm of the Hydrogen ion concentration, that’s why the formula to find pH is given by
![pH=-log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/nmx0o2p707irwdw9nb6k79qr3wnxve6l75.png)
or
![pH=-log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/rc7t49w29zldplnaie8e1v3wxlzlirtjbp.png)
![pH=-log[5.6*10^(-2) M]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/m4z0u6usotf03l8m0zyoz0wqye531t5386.png)
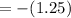
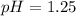
(Answer)
Please note:
When an hydrogen atom loses an electron, Hydrogen ion is formed, which will contain 1 positive proton and 0 negative electron and 0 neutral neutron.
Thus Hydrogen ion has a + charge.
Hydrogen ion is also called as a proton since it has only 1 proton in it.
Hydrogen ion in water that is,
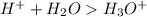
H_3 O^+ is called as Hydronium ion.