Answer:
The new pressure in the holding tank is 2 atm.
Step-by-step explanation:
Given that,
Volume = 10000 L
Initial pressure 2 atm
Initial temperature = 300 k
We need to calculate the new pressure
Using equation of ideal gas
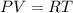
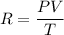
Where,P = pressure
T = temperature
V = volume
R = constant
Put the value into the formula
Volume of gas are remain constant
So,
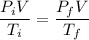
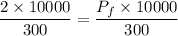
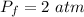
Hence, The new pressure in the holding tank is 2 atm.