Step-by-step explanation:
As it is given that value of equilibrium constant is
 .
.
And, it is known that if the value of K, that is, equilibrium constant is greater than 1 then the reaction is favorable and proceeds in the forward direction.
This means that formation of products is favored then. As a result, concentration of products will be more.
Whereas if value of K is smaller than 1 then reaction is unfavorable and proceeds in the backward direction. As a result, concentration of reactants will be more.
Since, it is given that K =
 which is greater than 1. So, it means reaction is proceeding in the forward direction. Hence, concentration of products which is
which is greater than 1. So, it means reaction is proceeding in the forward direction. Hence, concentration of products which is
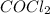 will be more.
will be more.
Thus, we can conclude that for the given reaction the equilibrium concentration of the reactants are much smaller as compared to the equilibrium concentration of the product.